QUALITY MANAGEMENT SYSTEM
Innovations brought by ISO 22000: 2018 FSMS
This standard, which lasts to 13 years to update, is still remains a system standard, especially compared to the standards preferred by exporting companies such as BRC, IFS, FSSC 22000, SQF, AIB. In fact, ISO 9001:2015 and 14001:2015 process started with the "High-Level Structure" feature by including the documentation improves itself. I guess it would not be wrong to say that it contains documentation for those who do not like to do unreal things, although there is not exactly documentation. First, let's take the positives about the standard:
• Monitoring, Verification and Validation in terms are clearly defined and leaves no question marks in the head.
• We meet a new term that finds a special place for the emotions that oscillate between CCP (Critical Control Point) and OPP (Operational Prerequisite Program). If the CCP has a "Critical Limit", OPP also has an "Action Criteria".
• Traceability, which we got used to with BRC and IFS, is now waiting to be implemented in the new standard.
• Be prepared to place sketches next to the flow chart (food and non-food areas, personnel, product, packaging, etc. are now waiting for the flow of the system).
• The Risk and Opportunity study, starting with ISO 9001: 2015, is also expected here, and when using the Organizational Risk definition for the desired Risks with this logic, HACCP and Hazard Analysis take place as Operational Risk.
• The beauty brought by the high-level structure is also in question in this standard. It wants to ensure continuous improvement (article 10) after first planning and implementing (articles 6 and 7) (article 8), analyzing data and measuring performance (article 9).
• Issues such as sabotage and adulteration are also elements that should be included in the emergency action plan.
• It is stated that Allergen and Radiological residue should also be taken into consideration in Food Safety Hazards.
• There is yet another new term "Major Food Safety Hazard". We can say that the danger that needs to be eliminated with these hazard control measures.
Although its relative downsides:
• Unfortunately, while customer terms and legal conditions are a necessity during the creation of PPs (Prerequisite Program), ISO 22002 series standards are the issues to be considered.
• The topic of context is again encountered in article 4, it is discussed how long it will take place in our country.
• Measuring devices (sources) mentioned in the support (article 7) part of ISO 9001: 2015, Critical Limits etc. in this standard. It is mentioned in article 8 on monitoring. This will be a bit complicated, especially for companies that install integrated systems.
ISO 9001: 2015 Standard Structure
0 Introduction
1 Scope
2 Cited standards and/or documents
3 Definitions and terms
4 Organization Structure
4.1 Understanding the setup and structure
4.2 Understanding the needs and expectations of interested parties
4.3 Quality management s determination of the scope of the claims
4.4 Quality management system and processes
5 Leadership
5.1 Leadership and commitment
5.2 Policy
5.3 Organizational roles, responsibilities and authority
6 Planning
6.1 Activities addressing risks and opportunities
6.2 Quality objectives and targets to achieve for planning
6.3 Planning of changes
7 Support
7.1 Resources
7.2 Competence
7.3 Awareness
7.4 Communication
7.5 Documented Information
8 Operation
8.1 Operational planning and control
8.2 Product and terms of service
8.3 Product and service design and development
8.4 Control of externally provided process, product and service?
8.5 Production and service provision
8.6 Release of product and service
8.7 Control of nonconforming output
9 Performance evaluation
9.1 Monitoring, measurement, analysis and evaluation
9.2 Internal audit
9.3 Management review of spend
10. Improvement
10.1 Nonconformity and corrective action
10.2 Continuous improvement
Standard Clauses Overview
0. Introduction
0.1 General
0.2 Quality Management Principles
0.3 Process approach
0.4 Risk-Based Thinking
0.5 Relationship with Other Management System Standards
1. Scope
2. Cited standards and / or documents
3. Definition and Terms
4. Installation Labor's Structure
It is clear that the scope of the QMS should be determined by taking into account the internal and external dimensions that may affect the organization's achievement of the intended QMS outputs and its strategic orientation and the conditions of the relevant parties.
In the establishment of the QMS, it is required to understand the conditions, needs and expectations of the related parties. There may be circumstances, legal and regulatory requirements or contractual requirements.
The organization should determine the necessary processes for the QMS. With the new revision, the organization should identify the risks and opportunities that the organization may face in determining and implementing the processes, providing the product and service (in other words, senior management should know the strengths and weaknesses of the organization and understand how it can affect the delivery of the product or service).
5. Leadership
5.1 Leadership and commitment
The conditions in this article are based on the concept of "Senior Management" in which the organization is represented at the highest level.
Senior management is responsible for establishing the quality policy and goals in line with the organization's structure and strategic orientation.
Senior management should identify risks and opportunities that may affect the suitability of the product and service and the organization's ability to increase customer satisfaction.
5.3 Quality Policy
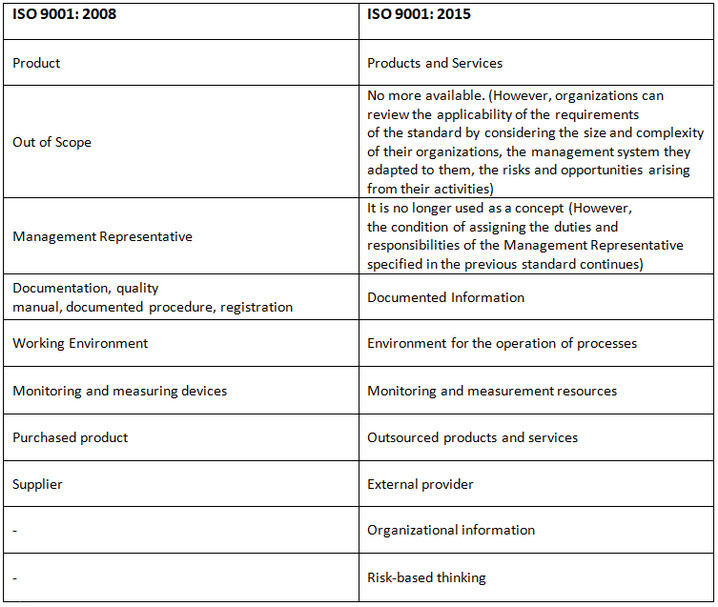
There is a reference to "Documented Information". Unlike the 2008 version, there is a requirement that relevant parties be available where it is applicable.
5.3 Organizational roles, responsibilities and authority
There is no direct definition of "Management Representative". It is required to determine the responsibilities and roles for two important tasks, which are the compliance of the QMS created within the organization with the ISO 9001 standard and the reporting of the performance of the QMS.
6. Planning
6.1 Activities addressing risks and opportunities
This clause replaced the "Preventive Action". While planning the quality management system, the organization should take into account the internal and external dimensions (4.1) and relevant parties (4.2),
" "To guarantee that the QMS reaches the desired results
" "To prevent/minimize undesirable effects
" "It should identify risks and opportunities for continuous improvement.
6.2 Quality objectives and planning to achieve targets should include;
Quality goals;
What to do?
What resources to use?
Who will be responsible?
When to complete?
How the results will be evaluated?
7.Support
7.1 Resources
The organization should determine the necessary resources for the establishment, implementation, maintenance and continuous improvement of the QMS.
Unlike the ISO 9001: 2008 revision;
Working Environment = 7.1.4 Environment for the operation of processes may include physical, social, psychological, environmental factors.
Article 7.1.5 Monitoring and Measurement of Resources "Documented Information" (Calibration)
7.5 Documented Information
The requirement to create a documented Quality Handbook and 6 mandatory document procedures has been removed. According to the new revision, organizations will decide;
"Its size and types of activities, processes, products and services.
"The complexity of processes and their interactions.
"How much and in what form they will create documented information according to the competence of the people.
Requirements referred to as "documented procedure" in ISO 9001: 2008 standard are "maintaining documented information" in the new revision.
In the ISO 9001: 2008 standard, the requirements referred to as "registration" are expressed as "storing the documented information".
8.Operation
8.1 Operational planning and control
The requirements of article 7.1 of ISO 9001: 2008 have been clarified. Differently, it is this article to control the external process.
8.2 Determination of product and service terms
It corresponds to the customer-related processes article. There is no major change. More detailed explanations are available.
8.3 Design and development of the product and service
Design review, verification and validation; Unified in control of design and development.
8.4 Control of externally provided product and service
It corresponds to article 7.4.1. Differently, the requirement of addressing the process of the external provider to provide the product and service directly to the customer has been added.
It can be considered as an outsourced process or function of an external provider. Evaluation, performance monitoring and re-evaluation = "Documented Information"
In addition to 7.4.1, clause 8.4.2 (control type and scope of external presentation) requires the consideration of the potential impact on the delivery of the product and service under the control of the external provider.
8.5.1 Control of product and service delivery
It corresponds to clause 7.5.1. Besides;
Competence and qualification of personnel
Validation and periodic validation (Special Process conditions)
"Documented Information" instead of direct reference to work order
Performing monitoring and measurement for process control is more clearly stated.
Use and control of the appropriate infrastructure and process environment
8.5.2 Identification and traceability = 7.5.3
8.5.3 Property owned by the customer and external provider
Ensuring control of the ownership of the external provider in addition to the customer property clause (Customer property includes customer supplied components, mold, equipment, land, personnel information, intellectual property, etc.)
8.5.4 Enclosure = 7.5.5
8.5.5 Post-Delivery Activities
It was considered as a separate article. In the ISO 9001: 1994 version, clause 4.19 was under 7.5.1 in the 2008 version. Legal Terms are included. (Warranty terms etc.)
8.5.6 Control of changes
In addition to the design and development changes, the requirement of control and review of unplanned changes has been added.
8.6 Release of product and service corresponds to clause 8.2.4.
8.7 Control of improper process output, product and service
Article 8.3 has been expanded. Clearer conditions have been introduced.
9.Performance Evaluation
9.1 Monitoring, measuring, analysis and assessment organizations QMS in
What’s,
What way (method),
When to monitor and measure and
Delta district must identify when analyzed and the results of that evaluation.
Quality performance and evaluation of the effectiveness of the quality management system are determined as new conditions.
9.1.2 Customer Satisfaction
Customer opinions and suggestions are clearly expressed.
9.1.3 Analysis and Evaluation = 8.4 9.2 Internal audit = 8.2.2
9.3 Management Review = 5.6 10th Improvement
10.2 Non-conformity and corrective action
8.3 and 8.5.2 have been collected under this clause.
Design by WEBINSA
Copyright © dknkalitedanismanlik.com